gravimetric analysis vs spectrophotometric method|Gravimetric Analysis: Definition, Principle, and Example : suppliers Gravimetric analysis is a quantitative method for accurately determining the amount of a substance by selective precipitation of the substance from an aqueous solution. . 4 de mai. de 2023 · Vídeos todos os dias às 10:00 e 17:00 horas! #estrategias #slots #ganhardinheiro #fortunerabbit #estratégias Esse vídeo é para maiores de 18 anos. Lembre-se sempre que toda .
{plog:ftitle_list}
Live-Action novo LANÇAMENTO! [Live Action Hentai] O Únic.
Principle and steps involved in the gravimetric analysis
“Analysis” menu and enter the appropriate absorbance values and concentrations of phosphate for each colored solution. The resulting Beer’s Law plot will be your calibration curve of A vs. concentration of Gravimetric analysis is a quantitative method for accurately determining the amount of a substance by selective precipitation of the substance from an aqueous solution. .An important part of the chapters that follow is a consideration of how we can use the emission or absorbance of photons to determine the concentration of an analyte in a sample. Here we .
Gravimetric analysis is a quantitative method used in analytical chemistry to determine the amount of a substance present in a sample by measuring its mass. This technique relies on the principles of precipitation and weighing to isolate .
Differences between volumetric analysis and gravimetric analysis. In contrast to volumetric analysis, which determines the amount of a desired element by measuring its volume, gravimetric analysis measures the .
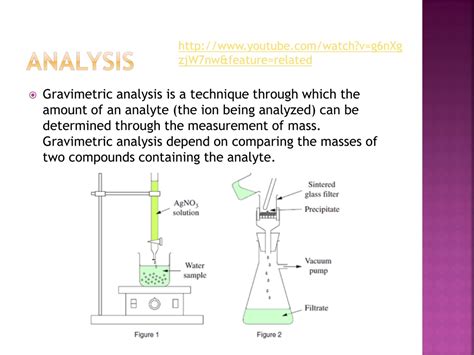
Types of Gravimetric Methods; Conservation of Mass; Why Gravimetry is Important; Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. Later, as you read .Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight.Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. The principle of this .
Gravimetric analysis describes a set of methods in analytical chemistry for the quantitative determination of an analyte based on the mass of a solid. A simple example is the .
A new, rugged, precise, accurate and fast primary method of measurement has been proposed for the determination of gold in various gold articles. Precise and accurate measurement of gold is the primary requirement for hall marking and to trade gold internationally, as billions of dollars of gold are trading world wide for the various applications. At present Fire . Studies of human blood are carried out for numerous parameters. One of them is sodium concentration. In this article, ion chromatographic, spectrophotometric, titrimetric, and gravimetric procedures for determination of sodium concentration in plasma, serum, and whole blood are described. Brief data on these procedures and results of their comparative tests are .
A general principle of gravimetric method of analysis is based on a chemical reaction between analyte and reagent. The analyte (A) of molecules ‘a’ react with the reagent (R) of molecule ‘r’. After drying, the product formed . Gravimetric testing is the most common and economical method used for pipette calibration. In this process, an aliquot of distilled water is placed in a vessel, and its weight is measured using an analytical balance. . This method uses light absorption to verify volume accuracy with a photometer and is used for low volume measurements and .Gravimetric Analysis vs. Titrimetric Analysis . and pharmaceutical research. Gravimetric analysis and titrimetric analysis are two widely used methods for determining the concentration or composition of substances in a sample. While both methods aim to achieve accurate results, they differ in terms of principles, procedures, and applications. .Here we provide a brief summary of quantitative spectroscopic methods of analysis, leaving more specific details for later chapters. This page titled 6: An Introduction to Spectrophotometric Methods is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by David Harvey .
Statistical analysis was applied in order to compare the proposed gravimetric analytical method with the standard gravimetric method. The correlation curve is described with the equation y=0.660+0.998 x. x and y denote the amount of piperazine in mg determined by the picric- and picrolonic-method, respectively. In the present work two new analytical methods, a gravimetric and a spectrophotometric, for the assay of piperazine free base and its soluble salts in the anthelmitic preparation "Oxyuran", are .
Gravimetric analysis: Steps,Types, Advantages,
Method 925.10 in Official Methods of Analysis, 18th Edition (AOAC International, 2007) provides an approved method for determining the moisture content of flour. A preweighed sample is heated for one hour in a 130 o C oven and transferred to a desiccator while it cools to room temperature. Sulfate concentrations are determined in mine water by gravimetric, titrimetric, colorimetric, turbidometric, ion chromatographic, inductively coupled plasma absorption spectrophotometric, and .Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection . Spectrophotometry is a tool that hinges on the quantitative analysis of molecules depending on how much light is absorbed by colored compounds. . This method is not very accurate since the composition of proteins . They found that the coefficients of molar extinction of proteins measured by gravimetric analysis are lower than those obtained using the Edelhoch method. The reasons why gravimetric analysis overestimates protein concentration compared to UV–Vis spectrometry was previously discussed by Pace et al. and Anders et al. (Pace et al., 1995, Anders .
Main Difference – Gravimetric vs Volumetric Analysis. The amount of a constituent present in a mixture of constituents can be determined using either gravimetric analysis or volumetric analysis. These methods can be used to determine the purity of a constituent in a given sample.
one of the common methods for determination of ethanol concentration in alcoholic beverages due to its accuracy, and sensitivity4. However, this method requires expensive instrument and skilled worker for analysis. In addition, one instrument could analyze one sample at a time of analysis. Distillation is one Gravimetric method was selected to prepare the standard oil solutions. According to the figures of merit, the introduced method had a wide linear response from 1.0 to 400.0 µg mL −1 for oil determination in . one can simply and rapidly compare the amount of oil in different samples by visual imaging besides the spectrophotometric analysis.The bubbling was due to the production of CO 2.. The test of vinegar with potassium carbonate is one type of quantitative analysis —the determination of the amount or concentration of a substance in a sample. In the analysis of . Classical Methods of Analysis; Modern Instrumental Methods of Analysis; Analytical chemistry has a long history. On the bookshelf of my office, for example, there is a copy of the first American edition of Fresenius's A .
In this method, the phytate was hydrolysed by the enzyme phytase coupled to a solid phase packed into an enzymatic reactor, and the obtained hydrolysed orthophosphate was determined by a spectrophotometric method dedicated to the analysis of Pi . Based on a similar idea, in 2016, McKei proposed acid extraction and determination of phytic acid . Method 925.10 in Official Methods of Analysis, 18th Edition (AOAC International, 2007) provides an approved method for determining the moisture content of flour. A preweighed sample is heated for one hour in a 130 o C oven and transferred to a desiccator while it cools to room temperature.
Because the release of a volatile species is an essential part of these methods, we classify them collectively as volatilization gravimetric methods of analysis. 8.4: Particulate Gravimetry Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participates in a chemical reaction. Total petroleum hydrocarbons (TPH) is an important parameter for evaluating risk and establishing cleanup requirements at petroleum release sites. However, different analytical methods may provide incomparable results. To select more appropriate method and design cost-effective remediation strategy, a comparison study of four analytical methods (gravimetric .Statistical analysis was applied in order to compare the proposed gravimetric analytical method with the standard gravimetric method. The correlation curve is described with the equation y=0.660+0.998 x. x and y denote the amount of piperazine in mg determined by the picric- and picrolonic-method, respectively. Spectrophotometric determination .

Gravimetric analysis is an analytical technique used for the quantitative determination of an analyte based on the mass of a solid. . Electro gravimetric method is employed to separate the ions of a substance, often a metal. In this method, the analyte solution is electrolyzed. As a result of the electrolytic reduction, the analyte is .
Most of the scientists have been using different spectroscopic and spectrophotometric techniques like Infrared spectroscopy, Raman spectroscopy, X-ray fluorescence and UV VIS spectrophotometry etc .
GRAVIMETRIC METHOD Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight.Recommended Beer Degassing Methods and Alternatives Matrix. The degassing of beer is a critical sample-preparation step for many beer analyses. Although multiple options exist for degassing, each has its own advantages and disadvantages, including degassing time, cost, throughput (one sample vs. multiple samples), release of volatiles (can negatively impact .AbstractTHIS text-book, written by one having considerable practical experience, will be welcomed by mineralogists and analytical chemists generally. It gives a critical description of modern gravimetric analytical methods in a very convenient form.Gravimetric Analysis: a Laboratory Manual with Special Reference to the Analysis of Natural Minerals and Rocks. By .
webCanal focado no tema cuckold com muitos prints, contos, humor, hq, fotos com legendas. Tudo organizado e etiquetado no canal. Venham conferir o melhor canal cuckold do telegram. Regras: Canal Do Telegram. ENTRAR NO GRUPO. Canal e .
gravimetric analysis vs spectrophotometric method|Gravimetric Analysis: Definition, Principle, and Example